Folate, colloquially known as Vitamin B9, stands as an indispensable nutrient and a foundational pillar of human biology. Its roles are elemental, facilitating DNA synthesis and regulating cellular repair. It also manages the detoxification of harmful metabolic byproducts. Yet, the common understanding of this essential vitamin is fraught with a critical misconception. This error pervades the medical literature. In public consciousness, synthetic folic acid is treated as synonymous with natural folate. This conflation ignores basic chemistry. This error is not merely semantic; it represents a profound biochemical misunderstanding with significant health consequences. For many, synthetic folic acid remains a metabolic challenge. It lingers unutilized in the bloodstream. Meanwhile, cells starve for the specific compound they truly require: activated folate.
This essay explores the critical distinction between folic acid and activated folate through the lens of modern biochemistry. The active form is scientifically known as 5-Methyltetrahydrofolate (5-MTHF). We examine the intricate metabolic pathways of the one-carbon cycle and the MTHFR genetic bottleneck. This builds a robust case for a new paradigm in nutritional science. It moves beyond the blunt instrument of mass fortification with folic acid. It favors a personalized, biochemically informed approach. The goal is providing the body with the bio-available Vitamin B9 it needs to thrive. The journey to a targeted nutritional strategy begins with a deep appreciation for this nutrient. This exploration illuminates why activated folate serves as the superior choice for optimal health. It bypasses the metabolic hurdles that render other forms of B9 ineffective for many.
Molecular Distinctions in the Vitamin B9 Family
To grasp the superiority of Vitamin B9 forms, one must understand the metabolic journey from ingestion to cellular function. The term “folate” refers to a family of compounds sharing a similar structural backbone, rather than a single molecule. In nature, dietary folates found in leafy green vegetables exist as complex polyglutamate structures. Upon consumption, the gut employs specific enzymes to deconjugate these chains into simpler monoglutamates, which are then absorbed into the bloodstream. However, this is only the beginning. Once absorbed, these natural folates must undergo a series of enzymatic conversions within the liver to be transformed into the final, universally usable form. This multi-step process, while natural, is where the first potential for inefficiency arises, as the body often struggles to produce sufficient activated folate to properly fuel its necessary biological systems.
The introduction of synthetic folic acid presents a far more significant metabolic challenge because it is chemically distinct from what the body expects. Folic acid is a fully oxidized, man-made molecule that does not occur anywhere in nature, synthesized primarily for its shelf-stability. It was selected as an ideal candidate for fortifying processed foods like flour to prevent deficiency diseases. To become biologically useful, however, folic acid must first be reduced by an enzyme called dihydrofolate reductase (DHFR). Herein lies the critical bottleneck that most medical professionals overlook. In humans, the activity of the DHFR enzyme is remarkably low, functioning several hundred times slower than in species like rats. Consequently, when the body encounters high doses of folic acid, the liver’s limited capacity is quickly overwhelmed, significantly halting the necessary production of activated folate.
The Metabolic Superiority of Active Folate
This enzymatic traffic jam leads to a problematic condition termed the accumulation of “Unmetabolized Folic Acid” (UMFA) in the systemic circulation. While the precise long-term health effects of high UMFA levels are still being investigated, research suggests potential links to masked Vitamin B12 deficiency and even an increased risk for certain cancers. More immediately, the presence of UMFA signifies a profound metabolic failure where the body possesses the wrong molecule in high amounts. The body is flooded with a precursor molecule it cannot efficiently process, while the cells, tissues, and organs that depend on a steady supply of methyl donors are left wanting. They are not waiting for folic acid; they are waiting for activated folate to drive their functions. Without this specific form, the cellular machinery grinds to a halt, leading to a cascade of inefficiencies that manifest as fatigue, cognitive brain fog, and systemic inflammation.
The unique power of supplementing directly with active folate lies in its ability to bypass this compromised DHFR pathway. As the primary form of folate found in blood plasma and the only form capable of crossing the blood-brain barrier, it enters cells directly, requiring no further enzymatic reduction. It is, by definition, the most biologically efficient and readily available form of Vitamin B9, making the conversation about its superiority to folic acid one of fundamental biochemistry rather than mere preference. Without a sufficient supply of activated folate, the body’s most critical processes, including neurotransmitter synthesis and DNA methylation, are compromised. Therefore, ensuring an adequate intake of this specific nutrient is essential. The diagram below illustrates the metabolic blockage that occurs with folic acid compared to the direct utilization pathway of 5-MTHF, demonstrating why the active form is vastly more efficient for cellular metabolism.
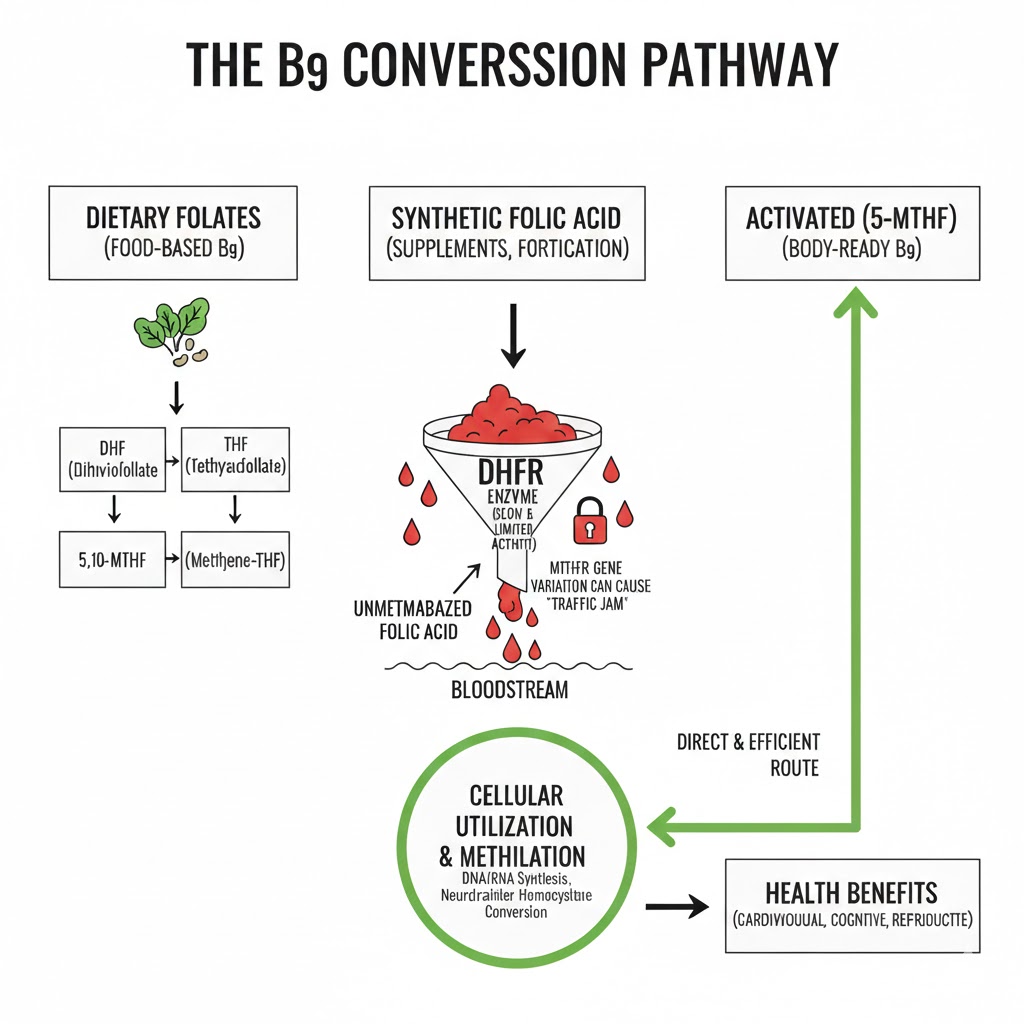
The Genetic Bottleneck of the MTHFR Mutation
The biochemical argument for prioritizing activated folate is powerfully reinforced by the insights gained from modern genomics and DNA sequencing. Widespread genetic testing has revealed that a significant percentage of the global population possesses common variants, or Single Nucleotide Polymorphisms (SNPs), in the MTHFR gene. This gene provides the essential blueprint for producing the methylenetetrahydrofolate reductase enzyme, the final and rate-limiting step in the conversion of folate into its active form, 5-MTHF. When this enzyme’s function is compromised due to genetic variance, the body’s ability to produce activated folate is dramatically reduced, regardless of the initial source of B9 intake. This means that even individuals eating a diet rich in leafy greens may still suffer from a functional deficiency if their genetic makeup hinders the final conversion step, necessitating an exogenous source of the active nutrient.
Two primary MTHFR SNPs are of clinical concern: C677T and A1298C, both of which affect the enzyme’s structure and thermal stability. The C677T variant is strongly associated with cardiovascular health and the regulation of homocysteine levels, acting as a major risk factor for heart disease. Individuals who are homozygous for this variant (possessing two copies of the gene) experience a staggering drop in MTHFR enzyme efficiency, often by as much as 70% compared to the wild type. The A1298C variant, meanwhile, is more closely linked to the production of neurotransmitters and consequently impacts mood and cognitive function. A person can be heterozygous (one copy) or homozygous for either variant, or be a compound heterozygote (one copy of each), all leading to varying degrees of reduced enzyme function. In all these scenarios, the body struggles to manufacture enough activated folate to meet systemic demands, creating a chronic deficit.
Systemic Consequences of Low Active Folate
The downstream consequences of this genetic bottleneck are systemic, severe, and affect nearly every organ system in the human body. A dysfunctional MTHFR enzyme stalls the entire “methylation cycle,” also known as one-carbon metabolism, which is the engine for cellular renewal. Methylation is a deceptively simple yet profoundly important biochemical process involving the transfer of a methyl group (one carbon and three hydrogen atoms) from one molecule to another. This transfer acts as a biological switch, turning genes on and off (epigenetics), repairing DNA, processing hormones, and detoxifying the body. When the production of activated folate is impaired due to an MTHFR mutation, this fundamental switch effectively rusts in place. Without the donation of methyl groups, the body loses its ability to regulate essential functions, leaving the individual vulnerable to accelerated aging and disease progression caused by unchecked oxidative stress and cellular damage.
Without an adequate supply of methyl groups derived specifically from activated folate, the body cannot properly convert the inflammatory amino acid homocysteine back into methionine. It cannot effectively synthesize neurotransmitters, nor can it manage gene expression or efficiently clear environmental toxins. Chronic inflammation, cardiovascular disease, and neurological dysfunction often follow as the methylation cycle slows down. For individuals with these genetic variants, consuming folic acid is akin to pouring crude oil into a car that requires refined gasoline; the tank is full, but the car will not run. The raw material is present, but the engine lacks the machinery to process it. Supplementing directly with activated folate is the only logical and effective solution, providing the finished fuel that the body’s compromised metabolic engine desperately needs. This makes a strong case for prioritizing this nutrient in all modern supplementation strategies.
Neuropsychiatry and the Role of Activated Folate
The recognition of activated folate as the body’s preferred form of B9 has catalyzed a revolution in clinical practice, particularly regarding mental health. Researchers and practitioners now leverage its unique properties to address several high-stakes health conditions where traditional therapies have failed. The shift from prescribing folic acid to utilizing 5-MTHF has demonstrated profound efficacy, particularly in the fields of neuropsychiatry and mood regulation. In the context of mental health, the brain has an immense demand for methyl donors to synthesize BH4 (Tetrahydrobiopterin), a critical cofactor required for the production of key neurotransmitters, including serotonin, dopamine, and melatonin. Without sufficient BH4, neurotransmitter synthesis plummets, leading to mood destabilization. This biochemical dependency explains why deficiencies in activated folate are so frequently observed in psychiatric patients.
In cases of treatment-resistant depression, where patients fail to respond to standard SSRI medications, the underlying issue is often a deficiency of active folate within the central nervous system. Standard antidepressants work by keeping existing serotonin in the synaptic cleft longer, but they do not help the brain produce more serotonin. If the brain is unable to manufacture serotonin due to a lack of methylation, the drug has nothing to work with. By providing 5-MTHF directly, which readily crosses the blood-brain barrier, clinicians can restore the necessary building blocks for neurotransmitter synthesis, often leading to significant improvements in mood and affect. Similarly, low folate levels are strongly correlated with age-related cognitive decline and brain atrophy. By supporting the maintenance of the myelin sheath that protects nerve fibers, activated folate plays a neuroprotective role, highlighting its vast therapeutic potential for long-term brain health and cognitive longevity.
Cardiovascular Protection via Active Folate
The cardiovascular benefits of activated folate are centered on its unique ability to regulate homocysteine, an amino acid that becomes toxic at high levels. Elevated levels of homocysteine in the blood act like a chemical abrasive, damaging the delicate endothelial lining of arteries and promoting the buildup of atherosclerotic plaque. This process is a primary driver of heart disease, stroke, and heart attack, independent of cholesterol levels. The methylation cycle is the body’s primary mechanism for clearing homocysteine, converting it back into the essential amino acid methionine, which is then used for tissue repair. This conversion is entirely dependent on a methyl group donated by activated folate. Without this donation, homocysteine pools in the blood, creating a pro-inflammatory environment that dramatically degrades vascular health over time.
In individuals with MTHFR mutations, this clearance process is crippled, leading to chronically high homocysteine levels that diet alone often cannot correct. Supplementing with activated folate directly fuels this conversion, effectively lowering homocysteine levels and mitigating cardiovascular risk. Clinical studies have demonstrated that 5-MTHF is significantly more effective than folic acid at lowering homocysteine in people with the C677T mutation. This offers a powerful, non-pharmaceutical intervention for preventing cardiovascular events. Furthermore, by improving endothelial function and reducing oxidative stress within the blood vessels, activated folate supports healthy blood pressure and overall circulatory resilience. The graph below visualizes the inverse relationship between 5-MTHF levels and plasma homocysteine, underscoring the direct impact this nutrient has on maintaining a healthy cardiovascular system.
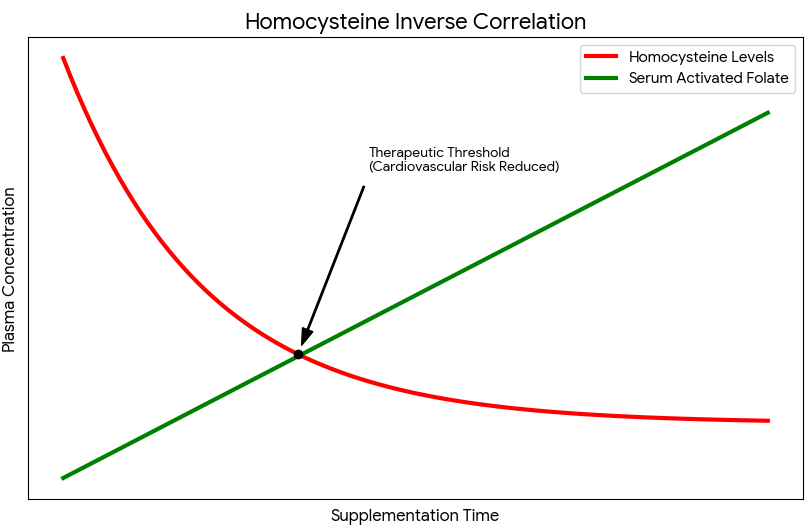 Prenatal Care and the Necessity of Activated Folate
Prenatal Care and the Necessity of Activated Folate
Perhaps the most well-known role for Vitamin B9 is in pregnancy, specifically for the prevention of neural tube defects like spina bifida. For decades, folic acid has been the standard of care recommended by governments and health organizations worldwide. However, the medical community is increasingly recognizing the superiority of activated folate for prenatal health, particularly given the prevalence of MTHFR mutations. By providing 5-MTHF, practitioners ensure that both mother and the developing fetus receive the bio-available methyl donors essential for healthy gene expression and cell division. This bypasses the risk associated with unmetabolized folic acid, which may block folate receptors in the placenta. The direct supply of activated folate ensures that the rapid cell division occurring in the first trimester proceeds without genetic errors or structural defects.
This direct supply of activated folate not only reduces the risk of neural tube defects but has also been linked to a lower risk of other pregnancy complications, such as preeclampsia and recurrent miscarriage. Research indicates that adequate methylation is required to vascularize the placenta properly; without it, placental function can be compromised, leading to poor fetal growth. Furthermore, because pregnancy places a massive demand on the mother’s folate reserves, women with MTHFR mutations are at a distinct disadvantage if they rely solely on folic acid. They may ingest the recommended amount but fail to convert enough to support two lives. Therefore, modern prenatal vitamins are increasingly replacing synthetic folic acid with activated folate, acknowledging that the benefits extend throughout gestation and offer a safer, more effective insurance policy for the health of the baby.
Fertility and the Impact of Active Folate
Beyond pregnancy itself, activated folate plays a crucial and often overlooked role in both male and female fertility. For women, adequate folate status is essential for ovarian function, egg quality, and the preparation of the endometrial lining for implantation. High homocysteine levels, caused by a lack of methylation, can lead to micro-clotting in the uterus, preventing successful implantation of the embryo. By optimizing methylation with the active form of B9, women can improve their reproductive environment. In men, the benefits are equally profound. Spermatogenesis is a process of rapid cell division that requires immense amounts of DNA synthesis. Studies show that men with MTHFR mutations often have lower sperm counts and higher DNA fragmentation rates, which are direct contributors to male factor infertility. Supplementing with activated folate has been shown to improve sperm parameters significantly.
The connection between methylation and fertility underscores the necessity of addressing nutrient forms before conception even occurs. Many couples struggling with “unexplained infertility” may actually be dealing with undiagnosed MTHFR mutations and subsequent folate deficiencies. In these cases, synthetic folic acid may be insufficient to correct the imbalance. Switching to a protocol that includes high-quality activated folate can resolve these metabolic roadblocks, lowering oxidative stress in the reproductive system and enhancing the viability of gametes. It represents a simple yet powerful intervention that addresses the root biochemical causes of reproductive struggles. As fertility science advances, the focus is shifting toward optimizing the parental biochemical terrain, with active folate serving as a cornerstone of pre-conception care protocols.
Detoxification Pathways and Active Folate
In an increasingly toxic world, the body’s ability to detoxify environmental pollutants is critical for maintaining health, and this process relies heavily on activated folate. The liver utilizes methylation to process and eliminate heavy metals, endocrine-disrupting chemicals, and excess hormones like estrogen. This is part of Phase II liver detoxification, specifically the methylation pathway. If this pathway is sluggish due to a lack of active B9, toxins accumulate in fatty tissues and the brain, contributing to chronic fatigue, autoimmune conditions, and hormonal imbalances. For example, the proper metabolism of estrogen prevents the buildup of harmful metabolites that are linked to breast and ovarian cancers. Activated folate provides the methyl groups necessary to convert these dangerous estrogens into safe, water-soluble compounds that can be excreted.
Furthermore, the production of glutathione, the body’s “master antioxidant,” is intrinsically linked to the methylation cycle. While glutathione production is technically part of the transsulfuration pathway, this pathway is downstream from the methylation cycle. If methylation is stalled due to a lack of activated folate, the production of glutathione is often down-regulated as a compensatory mechanism. This leaves the cells vulnerable to oxidative damage and impairs the immune system’s ability to fight off infections. By restoring the methylation cycle with 5-MTHF, one indirectly supports glutathione synthesis, thereby enhancing the body’s overall detoxification capacity. This highlights why active folate is not just a vitamin for DNA, but a critical component of the body’s waste management and defense systems.
A Practical Framework for Activated Folate Supplementation
Understanding the biochemical and genetic rationale for activated folate is the first step; implementing it safely and effectively is the next crucial phase. The market for B-vitamins can be confusing, saturated with conflicting labels and varying formulations, but a few key principles can guide individuals toward optimal supplementation. The very source of B9 matters immensely to the biological outcome. While leafy greens provide natural folates and are essential for overall health, achieving therapeutic levels through diet alone can be difficult for those with genetic variants. Synthetic folic acid, given its conversion issues and the prevalence of MTHFR mutations, should be actively avoided by those seeking to optimize their health. The most effective choice is a high-quality supplement providing activated folate, which is most often labeled as L-Methylfolate, L-5-MTHF, or 6(S)-5-MTHF.
When selecting a supplement, quality and the specific isomeric form are non-negotiable factors. The “L” or “6(S)” prefix is critical, as this indicates the biologically active isomer that fits into the body’s receptors. The “D” form is a mirror-image molecule that is biologically inert and may even compete with the active form for absorption, reducing efficacy. Reputable supplements often use patented forms like Quatrefolic® (a glucosamine salt) or Metafolin® (a calcium salt), which have been clinically studied for their stability, solubility, and bioavailability. These patented forms ensure the delivery of pure activated folate that resists degradation in the stomach and enters the bloodstream intact. Consumers must be vigilant in reading labels, as many “complex” formulas still “fairy dust” with cheap folic acid, undermining the benefits of the active ingredients.
Dosing Strategies for Active Folate
Transitioning to this potent nutrient requires a cautious and personalized approach, as individual responses can vary significantly. Because activated folate is so efficient, it can rapidly kick-start dormant methylation pathways, a phenomenon that can lead to symptoms of “over-methylation” in sensitive individuals. These symptoms might include anxiety, irritability, headaches, or insomnia, caused by a sudden surge in neurotransmitter production and detoxification. The guiding principle for safe supplementation is to “start low and go slow.” Beginning with a low dose (e.g., 400 mcg) allows the body to adapt to the influx of methyl groups without overwhelming the system. Dosing should be monitored and adjusted gradually over weeks, preferably under the guidance of a knowledgeable healthcare professional who understands methylation genetics.
For those with significant MTHFR blockages or high homocysteine, higher doses may eventually be necessary, sometimes reaching 1mg to 15mg per day, but this must be titrated carefully. It is also important to note that lifestyle factors, such as stress and diet, deplete activated folate levels, meaning that dosing requirements may change over time. Monitoring symptoms is key; if agitation occurs, niacin (Vitamin B3) can be used to “mop up” excess methyl groups, acting as a brake on the system. This nuance in dosing highlights that activated folate is a powerful metabolic intervention, not merely a passive multivitamin ingredient. Understanding this potency allows users to harness its benefits while minimizing potential side effects, creating a tailored approach to long-term wellness.
The Synergistic Role of B12 and Activated Folate
Finally, one must never consider activated folate in isolation, as its function is inextricably linked with Vitamin B12. Folate and B12 are synergistic partners in the methylation cycle, working in tandem to convert homocysteine to methionine. The enzyme methionine synthase requires B12 (in the form of methylcobalamin) to accept the methyl group from folate. Taking high doses of activated folate without ensuring adequate B12 status can lead to a condition known as the “folate trap.” In this scenario, folate becomes metabolically stuck inside cells, unable to complete its cycle because B12 is not present to accept the methyl group. This effectively renders the folate useless despite high blood levels, halting the methylation process.
Moreover, supplementing with folate alone can mask the hematological signs of a B12 deficiency (macrocytic anemia) while allowing the neurological damage associated with B12 deficiency to progress unchecked. This can lead to serious, sometimes irreversible, nerve damage. Therefore, any supplementation protocol with activated folate must be paired with an active form of B12, such as methylcobalamin or adenosylcobalamin, to maintain the delicate synergy of the methylation cycle. Avoiding cyanocobalamin, the synthetic form of B12, is also recommended for the same reasons one avoids folic acid; it requires conversion steps that can be inefficient. By combining active folate with active B12, one ensures the smooth operation of the methionine synthase enzyme, protecting both the blood and the brain.
Conclusion: The Biological Necessity of Activated Folate
The evidence compelling a shift toward activated folate is overwhelming and grounded in rigorous biochemical science. It represents a necessary move away from the imprecise and often ineffective strategy of mass folic acid fortification and toward a future of personalized, genetically informed medicine. By understanding our individual biochemistry, testing for MTHFR variants, and prioritizing the use of the body’s finished product—activated folate—we can bypass metabolic hurdles, support brain health, protect the cardiovascular system, and unlock a new level of cellular wellness. The superiority of activated folate is not a matter of opinion, but of biological fact supported by clinical outcomes. It is the key that fits the intricate lock of human methylation, making it the definitive choice for modern health and vitality.
A consistent supply of this nutrient is a cornerstone of this new approach to preventative health. Whether for a pregnant mother, a patient with depression, or someone seeking to reduce heart disease risk, the solution lies in respecting the body’s enzymatic limitations. The clinical recommendations are clear: for cellular delivery, efficient detoxification, and optimal gene expression, the body needs activated folate. By embracing this bio-identical nutrient, we align our supplementation strategies with our biology, ensuring that we are not just surviving, but providing our cells with the precise tools they need to flourish.

5 thoughts on “Activated Folate, 5-methyltetrahydrofolate (5-MTHF)”
Comments are closed.